Dittenhafer-Reed Research Group
Understanding mitochondrial function in human health and disease
Mitochondria, the cell’s powerhouse, exist at the center of cellular biosynthetic pathways and play a major role in energy production, controlling cell death and oxidative stress. Defects in mitochondrial function cause a multitude of inherited human diseases and contribute significantly to age-related pathologies, like neurodegenerative disorders and cancer. Work in Dr. Dittenhafer-Reed’s lab combines biochemistry, molecular biology and cell biology approaches to study the basic biochemical mechanisms governing mitochondrial function and metabolism to allow for a deeper understanding of these diseases.
Mechanisms of control of mitochondrial DNA transcription
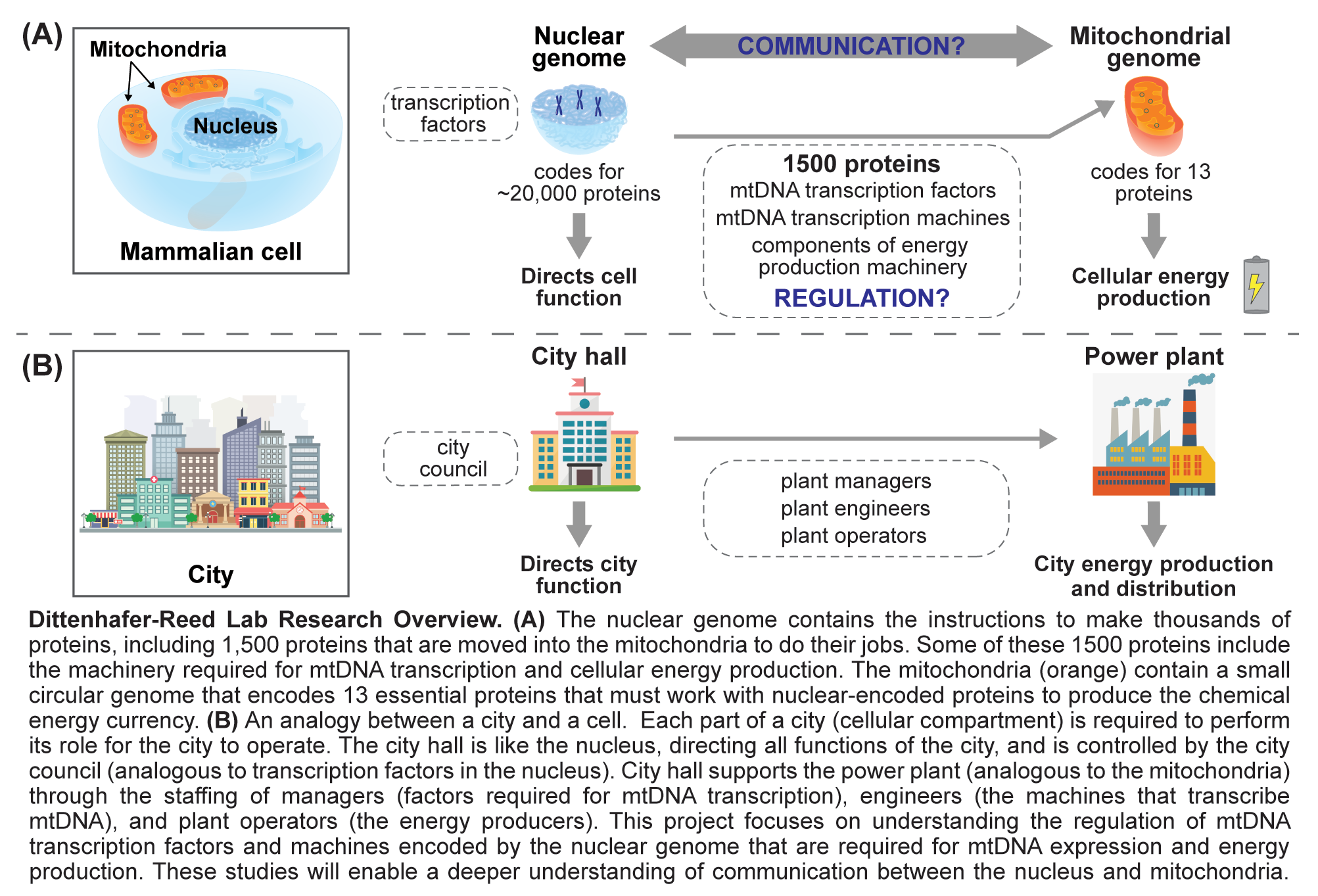
The nearly 10 million billion mitochondria found in the human body arose nearly two billion years ago when a eukaryotic cell precursor engulfed an energy producing proteobacterium. This proteobacterium evolved into the modern mitochondrion, known for producing the majority of cellular energy currency in the form of ATP. Over time, the mitochondrion has acquired a number of new functions central to cellular existence. Interestingly, mitochondria have retained their own small circular genome with single mammalian cells possessing hundreds of copies of mitochondrial DNA (mtDNA), a 16.6 kilobase circular double stranded molecule encoding 13 essential subunits of electron transport chain complexes. The rest of the approximately 1,500 member mitochondrial proteome, including an additional 70 oxidative phosphorylation components and all of the machinery required for mtDNA replication, transcription and translation, is encoded by the nuclear genome, which is inherited and treated independently of the mitochondrial genome. Therefore, coordination of nuclear and mitochondrial gene expression is essential for mitochondrial function. While core components of mitochondrial transcription initiation are known, a detailed understanding of transcriptional control is lacking. A goal of the research in the Dittenhafer-Reed lab is to uncover biochemical mechanisms that govern mitochondrial gene expression and mtDNA stability.
Characterization of mtDNA nucleoid proteins in genome maintenance and transcriptional regulation
The goal of our research is to uncover basic biochemical mechanisms that control mitochondrial gene expression and allow cells to respond to varying energetic demands. Our objective is to determine whether reversible protein modifications regulate mtDNA transcription. DNA carries the instructions needed to make the proteins found in the cell. Transcription is the process by which a certain piece of DNA (a gene) is selected for protein production. The process of translation follows and involves the synthesis of the protein using the transcribed DNA (mRNA) as a template. Every three nucleotides of the DNA carries the code for one amino acid. Amino acids join together in a chain and fold into a three-dimensional structure to yield a protein. Post-translational modifications are additional chemical decorations on the amino acids that comprise the protein that can be added and removed to regulate protein function. In some cases, the presence or absence of the chemical tag acts as a light switch, turning protein function on or off. In other cases, these modifications behave more as a dimmer switch, fine-tuning protein function up or down depending upon cellular needs.
Analogous to chromosomal DNA that wraps around histone proteins to fit in the nucleus, mtDNA molecules are compacted into mtDNA-protein complexes termed nucleoids. Mitochondrial nucleoid proteins compact and protect mtDNA and impact mtDNA transcription. Mass spectrometry studies identified nearly 60 proteins in nucleoid complexes. These proteins include the core transcriptional machinery, the factors required for mtDNA replication and translation and mitochondrial metabolic enzymes. A number of nucleoid proteins were identified to be post-translationally modified; however, much remains to be explored regarding the functional significance of these modifications. To understand the maintenance and gene expression of human mtDNA, the Dittenhafer-Reed lab uses biochemical approaches to analyze nucleoid protein structure, post-translational modifications and their functions.
A. Paul Schaap Science Center35 East 12th StreetRoom 3101Holland, MI 49423
workP. 616.395.7630
chemistry@hope.edu